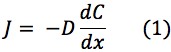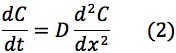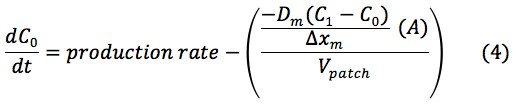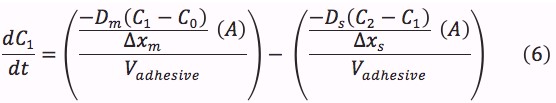iGEM REPORT: The Subtilis Defense: Developing a transdermal patch to deliver Bowman-Birk protease inhibitor peptide synthesized in situ to protect against ionizing radiation
Note: This iGEM Report was submitted to the PLOS iGEM Realtime Peer Review Jamboree, and has not undergone formal peer review by any of the PLOS journals. We welcome your comments on this work.
The Subtilis Defense: Developing a transdermal patch to deliver Bowman-Birk protease inhibitor peptide synthesized in situ to protect against ionizing radiation
Rachelle Varga (1), James Johnston (2), Siddhartha Goutam (2), Nilesh Sharma (3), Tiffany Dang (4), Nishi Patel (1), Nicholas D’Aleo-Sotas (1), Noshin Karim (4), Miriam Li (2), Syed Jafri (2), Elena Fekete (1), David Nguyen (4), Christine Phan (1), Neliza Mendoza (4), Shalpinder Dhothar (1), Daniel Ziemianowicz (2), Nicholas Jette (2), Anders Nygren (4), Darren Derksen (5), Mayi Arcellana-Panlilio (2) *
- Department of Biology, University of Calgary
- Cumming School of Medicine, University of Calgary
- Department of Neuroscience, University of Calgary
- Schulich School of Engineering, University of Calgary
- Department of Chemistry, University of Calgary
*Corresponding author: Mayi Arcellana-Panlilio (myarcell@ucalgary.ca)
Author Contributions
Conceptualization: RV JJ SG NS TD NP NDS NK ML SJ EF DN CP NM SD DZ AN DD MAP
Investigation: RV JJ SG NS TD NP NDS NK ML SJ DN CP NM
Formal Analysis: NS NK NM
Visualization: JJ NDS SJ CP
Writing – Original Draft: RV JJ SG NS TD NP NDS NK ML SJ EF NM
Writing – Review & Editing: RV JJ SG NS TD NP NDS NK ML SJ EF NM SD DZ MAP
Funding Acquisition: RV JJ SG TD NDS ML SJ DN DZ AN DD MAP
Project Administration: AN DD MAP
Supervision: DZ NJ AN MAP
Abstract
Exposure of astronauts to high-energy ionizing radiation is a substantial barrier to long-term space travel. Ionizing radiation can induce damage in DNA in the form of mutagenic double-stranded breaks, increasing an individual’s lifetime risk of cancer. Current solutions to prevent DNA damage during long-term space missions are ill suited.
The 2016 University of Calgary International Genetically Engineered Machine Team developed a conceptual design to address this issue. The goal was to engineer B. subtilis WB800 to express a modified peptide derived from the Bowman-Birk Protease Inhibitor, a protein natively found in soybeans that has been shown to elicit radioprotection in murine models. Engineered bacteria would be contained within a patch with growth medium and adhered to the surface of the skin. Allowing for the continuous production, secretion, and diffusion of the Bowman-Birk peptide from the patch for transdermal delivery into the body via the bloodstream.
Several key elements were tested. The radioprotective effects of the peptide were measured in 1BR3 human primary fibroblasts using immunofluorescent staining for ɣ-H2AX and 53BP1 (markers of DNA damage). B. subtilis growth in patch-like conditions was observed. Patch materials were tested to ensure bacteria would be contained while the peptide could flow through. The modified peptide was found to decrease the markers of DNA damage by up to 32%. B. subtilis was successfully maintained under patch-like conditions for three days. A size-selective membrane was successful in segregating the bacteria in the patch.
Financial Disclosure
Funding was generously provided by the University of Calgary, the Cumming School of Medicine, the Schulich School of Engineering, the Department of Biological Sciences at the University of Calgary, the University of Calgary Students’ Union, Mindfuel, and geekStarter (supported by Alberta Innovates Technology Futures). Materials were graciously donated by Dow Corning, 3M, Omega Biotek and Integrated DNA Technologies.
No listed funding organizations had any role in the planning, design or execution of our experiments. All aspects of this scientific manuscript were the product of the 2016 University of Calgary International Genetically Engineered Machine (iGEM) team. No counsel was accepted from the aforementioned funding organizations.
Competing Interests
The authors have declared that no competing interests exist.
Data Availability
All data is fully available without restriction at http://2016.igem.org/Team:UofC_Calgary.
Introduction
Overcoming damage incurred from ionizing radiation is a substantial challenge in the field of long-term space exploration.[1] With every trip into space, astronauts are exposed to high levels of X-rays and gamma rays, which can damage a cell’s DNA and lead to harmful mutations or cell death. As a result, the amount of time an individual may spend in space is currently capped at a lifetime maximum of three years.
Ionizing radiation (IR) disrupts biological molecules by breaking chemical bonds. In cellular DNA, IR can lead to highly mutagenic double-stranded breaks, which, if left unrepaired, will lead to cell death.[2] The cell’s natural response to double-stranded breaks is to activate the non-homologous end joining (NHEJ) pathway, in which two blunted ends of DNA molecules are fused together in a sequence-independent manner. NHEJ is inherently an error-prone process; therefore, the more double-stranded breaks an individual is subjected to, the higher the probability that errors or mutations will arise, increasing an individual’s lifetime risk of cancer.[2] Sources of ionizing radiation include the sun and the galactic cosmic rays. Humans are normally protected from ionizing radiation by the Earth’s magnetic field, however, little protection is offered outside of the magnetosphere.[3]
Current methods to protect astronauts from IR rely heavily on physical shielding. While effective, these methods are often expensive, difficult to employ, and not portable.[3] Lead or water shielding, for example, increases the cost of space missions dramatically; for every extra pound of material that must be transported into space, $10,000 USD of extra fuel must be consumed.[4] Other radioprotective methods include the use of space suits and radiation shelters made from radioprotective materials combined with minimizing IR exposure time, but these technologies are still in development, and will quickly become a limitation for missions in deep space.[3]
The Bowman Birk protease inhibitor (BBI) is a soybean-derived protein that has been shown to enhance the rate of NHEJ. This is mediated through the phosphorylation and nuclear transport of the epidermal growth factor receptor (EGFR) and subsequent recruitment of DNA repair machinery.[5] 10 μM of BBI in human primary fibroblasts is enough to elicit a relative radioprotective effect, a trend observed in multiple cell lines with functional TP53 proteins.[6] Furthermore, it has been shown that the radioprotective effects of BBI begin to plateau at 30 μM.[7] We propose that the administration of BBI could provide an additional level of protection to astronauts against IR, providing increased safety during space travel.
Transdermal patches are a non-invasive and portable method of administering drugs over long periods of time.[8] Here, an adhesive patch was designed for the delivery of peptide across the skin into the bloodstream. This patch would incorporate genetically engineered B. subtilis to continuously express and secrete a modified nonamer of BBI (mBBI) with the same capability for radioprotection for transdermal delivery.[7]
B. subtilis WB800 was chosen as a bacterial chassis for four unique properties: (1) B. subtilis WB800 is deficient in eight extracellular proteases, and would reduce the degradation rate of mBBI; (2) B. subtilis is capable of endospore formation, which would allow the transformed strain to remain dormant within the patch until activation by the user; (3) B. subtilis contains a native secretion pathway which can be taken advantage of to secrete mBBI directly into surrounding media; and (4) B. subtilis is naturally competent and has the ability to uptake foreign DNA when its comK gene, the master competency regulator, is activated.[9,10,11,12]
The relative efficiency of mBBI-mediated DNA repair was characterized via measurement of γH2AX and 53BP1 foci. These foci form proximal to sites of double-stranded breaks and are removed post-repair.[13,14] When damage occurs, H2AX histones of chromatin are phosphorylated to become γH2AX, a target for DNA repair complexes. A subunit of one of these complexes, 53BP1, can be stained via immunofluorescence. These foci can be visualized, serving as discrete indicators of the number of double-stranded breaks in a cell over time.[15]
The patch design considerations for use in space included long-term storage at ambient temperature, inexpensive to produce and transport, ease of use, and creating minimal waste. Thus, the transdermal patch was designed to be lightweight and portable and containing four layers: (1) a biocompatible backing layer capable of gas exchange; (2) a central pocket for bacterial growth and peptide production with four side pockets, one to isolate the bacterial spores prior to activation, and three to provide additional media for bacterial growth; (3) a size- and rate-controlling membrane with adhesive; and (4) a backing liner removed prior to application.
Materials and Methods
Truncated BBI Design
The experiments conducted in this study involve the truncated nonamer of BBI. To increase the solubility of the nonamer, the KSCI peptide sequence was added to the N-terminus, while a single phenylalanine was added to the C-terminus. The human influenza hemagglutinin (HA) tag was fused to the new C-terminus for immunodetection, giving a final peptide sequence 23 amino acids in length (KSCICALSYPAQCFYPYDVPDYA). This sequence will herein be referred to as modified BBI (mBBI). All peptides were synthesized by BioBasic.
Construction of Genetic Circuits
Six genetic circuits were designed for the production and secretion mBBI from B. subtilis WB800. In addition to the sequence for mBBI, several tags were added upstream and downstream of the peptide. A B. subtilis secretory tag and a transdermal tag from the USTC China 2013 iGEM team to allow for diffusion of mBBI across the skin were fused to the N-terminus.[16] Three of the constructs had mBBI fused to the N-terminus of GFP for visual detection.
Two constructs were designed for the expression of comK. The comK gene was flanked by sequences for the AmyE locus in the B. subtilis chromosome to allow for integration into the B. subtilis genome. All the designed sequences, except for comK circuits, contained PVeg, a constitutive B. subtilis promoter, and a ribosome binding site.
Full sequences of all inserts can be found online.[17]
Cloning
All genetic inserts were cloned into the pSB1C3 backbone. Synthesized genetic circuits for mBBI were cloned into in Escherichia coli TOP10 (New England Biolabs) using the iGEM BioBrick standard prefix and suffix. Both the vectors and inserts contained the standard biobrick prefix and suffix sequences. Double enzyme digests were performed to ensure proper directionality of inserts. DNA was cut using the restriction enzymes EcoRI, XbaI, PstI, and SpeI. Detailed protocols can be found online.[18] Competent E. coli TOP10 cells were transformed with the genetic circuits ligated into pSB1C3 using a standard chemical transformation protocol. The details of the protocol can be found online.[18]
B. subtilis Growth Curves
To simulate a 24-hour period of growth in an 8-hour day, three different cultures of B. subtilis WB800 were inoculated at various time points. The first set of three cultures was inoculated at 04:40, 04:45, 04:50 the day before the experiment was conducted; the second set of cultures was inoculated at 12:20, 12:25, and 12:30 the day of the experiment. The last set of cultures was inoculated at 08:00, 08:05: and 08:10 the day of the experiment. 1 mL of B. subtilis WB800 overnight culture was subcultured in 9 mL of media for each time point. To measure bacterial growth, optical density readings were taken at a wavelength of 600 nm using a spectrophotometer (Molecular Devices). Cultures were left to incubate while shaking at 200 rpm. An initial growth curve experiment was performed in Luria-Bertani broth (LB) containing 100 μg/mL hygromycin at three different temperatures: 4°C, 25°C, and 35°C. A second growth curve experiment was performed at 35°C using a variety of media, all containing 100 μg/mL hygromycin: LB, a 2X concentration of LB (2XLB), and Super Rich (SR).[19] During this second growth curve experiment, 1 mL of each type of medium was added to the cultures every 12 hours for the first 36 hours (a total of three times).
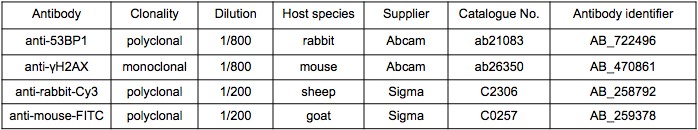
Immunofluorescence Tagging of γH2AX and 53BP1 Foci for Double-Stranded Break Detection
1BR3 human primary fibroblasts were generously provided by Dr. Aaron Goodarzi (University of Calgary) on July 15th, 2016.[20] They were cultured on glass coverslips (VWR International) in Minimal Essential Medium (Gibco) supplemented with 10% fetal calf serum and 10% Pen-Strep. Upon reaching confluency, cells were treated with 30 µM mBBI and irradiated with 2 Gy gamma radiation from a 137Cs source Gammacell 1000 irradiator (MDS Nordion) after 6 hours. Cells were subsequently fixed with 3% (w/v) paraformaldehyde containing 2% (w/v) sucrose. γH2AX and 53BP1 foci were visualized by immunofluorescence using an anti-γH2AX primary antibody (Abcam mouse) and an anti-mouse-FITC secondary antibody. 53BP1 foci were visualized using anti-53BP1 antibody (Abcam rabbit) and anti-rabbit-Cy3 secondary antibody. A complete list of antibody information can be found in Table 1. Nuclei were labeled with 4’,6-diamidino-2-phenylindole (DAPI). Coverslips were placed on slides using Vectashield mounting medium and the foci were examined under an oil immersion epifluorescent microscope (Axiovert 200) using a 63x lens (Zeiss). Foci over at least 40 cells were manually counted by individuals blinded to the IR and BBI treatments used.
Transdermal Patch Design
The patch was designed to contain a rectangular drug reservoir with the dimensions of 7 cm x 7 cm x 0.29 cm (width x length x height) with a volume capacity of 10 mL. Four packets are integrated into each corner of the patch, with three containing 1 mL of super rich media each, and the fourth containing desiccated B. subtilis WB800 spores.
The patch will be comprised of four layers as shown in Figure 1. The outermost backing layer will consist of CoTran™ 9722 Backing Polyethylene Monolayer Film (3M). This polyethylene material is breathable, heat sealable, and resistant to excipient and drug uptake. It will provide structural support of the patch and contain bacteria and media. The second layer, the drug membrane, will consist of CoTran™ 9728 Ethylene Vinyl Acetate Membrane (3M). This rate-controlling membrane composed of ethylene vinyl acetate (EVA) is responsible for control of the peptide diffusion rate, while containing bacterial cells in the drug reservoir. To ensure containment of the bacteria within the patch, a 0.2-micron filter membrane (Pall Corporation), placed between the second and third layers, will be tested. The third layer, the adhesive layer will consist of BIO-PSA Silicone-based Adhesive 7-4201 (Dow Corning). This layer adheres the patch to the surface of the skin. It also serves as an interface between the patch and skin to increase peptide diffusion. This product has high oxygen/gas permeability, low pain upon removal to sensitive skin, and increased diffusivity. The bottom layer, the release liner, will consist of Scotchpak 9755 Fluoropolymer Coated Polyester Film (3M), which protects the adhesive from being exposed to external factors. It is chemically inert to drug penetration and water. Materials used in patch development were generously donated by 3M and Dow Corning.

Patch Diffusion Assay
E. coli TOP10 transformed to express recombinant Green Fluorescent Protein (GFP) in a pSB1C3 plasmid backbone were used to test different materials for the drug membrane layer of the patch. 5 mL of an overnight culture grown in 5 mL LB containing 30 μg/mL chloramphenicol was drawn into a 10 mL syringe. LB media containing 30 μg/mL chloramphenicol was used as a negative control. Two membranes were tested and attached to the open end of each syringe: a CoTran™ 9728 Ethylene Vinyl Acetate Membrane (3M) and a 0.2-micron filter sterilization membrane (Pall Corporation). The syringes were placed in 250 mL Erlenmeyer flasks containing 10 mL of 0.9% (w/v) saline solution and covered with Parafilm. The flasks were left at room temperature for 24 hours. 150 μL of the overnight saline solution were plated on 1.5% LB-agar plates containing 30 μg/mL chloramphenicol. Plates were incubated at 37°C overnight. Plates were checked for signs of growth using UV light following a 24-hour incubation period. The measurements were repeated every 24 hours for seven days.
Patch Diffusion Modelling
MATLAB (Matrix Laboratory) was used to develop a diffusion model to numerically represent the diffusion of mBBI from the patch, through the skin and into the blood. This diffusion model was developed to answer the following questions:
- Does the peptide reach a constant concentration in the blood while the patch is on the user? If so, how long does it take?
- Literature values show that the minimum required amount of peptide needed for radioprotection is 10 μM.[6] Does the concentration in the blood reach 10 μM or higher?
Using Fick’s Law of Diffusion, the flux of mBBI diffusion from the patch to the bloodstream is represented by the following equations:
Where:
Thus, the change in concentration in the patch, skin and blood over time could be represented by the following equations:
The values of the variables in (4), (6) and (8) are located in Table 2.
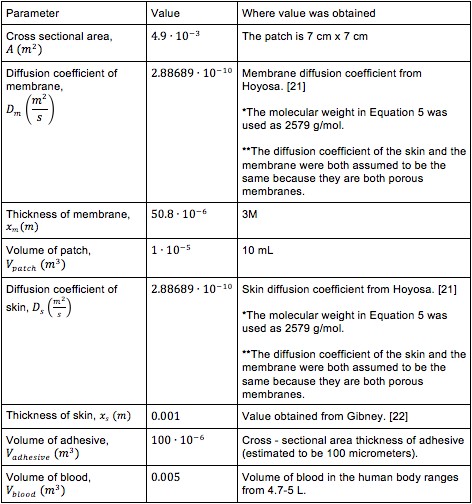
It was not possible to find literature values for the diffusion of mBBI through the patch’s size- and rate- controlling membrane and the skin. For this reason, it was assumed that the diffusion coefficient of the skin and size controlling membrane was 2.88689Ÿ10-10 m2/s as calculated from equation (5).[21]
The production rate, which is the amount of mBBI produced by B. subtilis in a given period of time, was assumed to be 1 mg/L.[23]
The degradation rate, which is the amount of mBBI lost in a given period of time through enzyme degradation in the liver and excretion through the kidneys, was determined by the following equation:
Where:
The exponential decay equation was used to determine the decay constant:
Equation (10) was rearranged for k:

The values used to solve (11), which used the half-life conditions for the peptide, can be found in the Table 3.
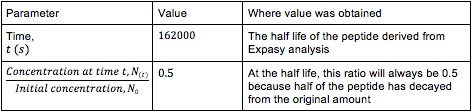
After solving for k, we found that its value was 4.2786863 x 10-5 s-1. Thus, equation (9) can be rewritten as:
![]()
Results
Determination of Patch Longevity
The B. subtilis WB800 growth curves at 4°C, 22°C, and 35°C are shown in Figure 2; the greatest amount of growth was observed in the bacteria incubated at 35°C. The exponential phase of growth occurred from 0 to 8 hours, while the stationary phase of growth began 8 hours after inoculation. B. subtilis WB800 growth peaked at an optical density of 4.04 at 15 hours post inoculation. Minimal decrease in optical density was observed after 18 hours.

At 22°C, the exponential phase was observed from 0 to 18 hours. Optical density reached a peak at 3.32 when growth leveled off in the stationary phase after 18 hours. After 24 hours, the optical density reached the same value as the culture grown at 35°C. No substantial growth was observed in cultures grown at 4°C.
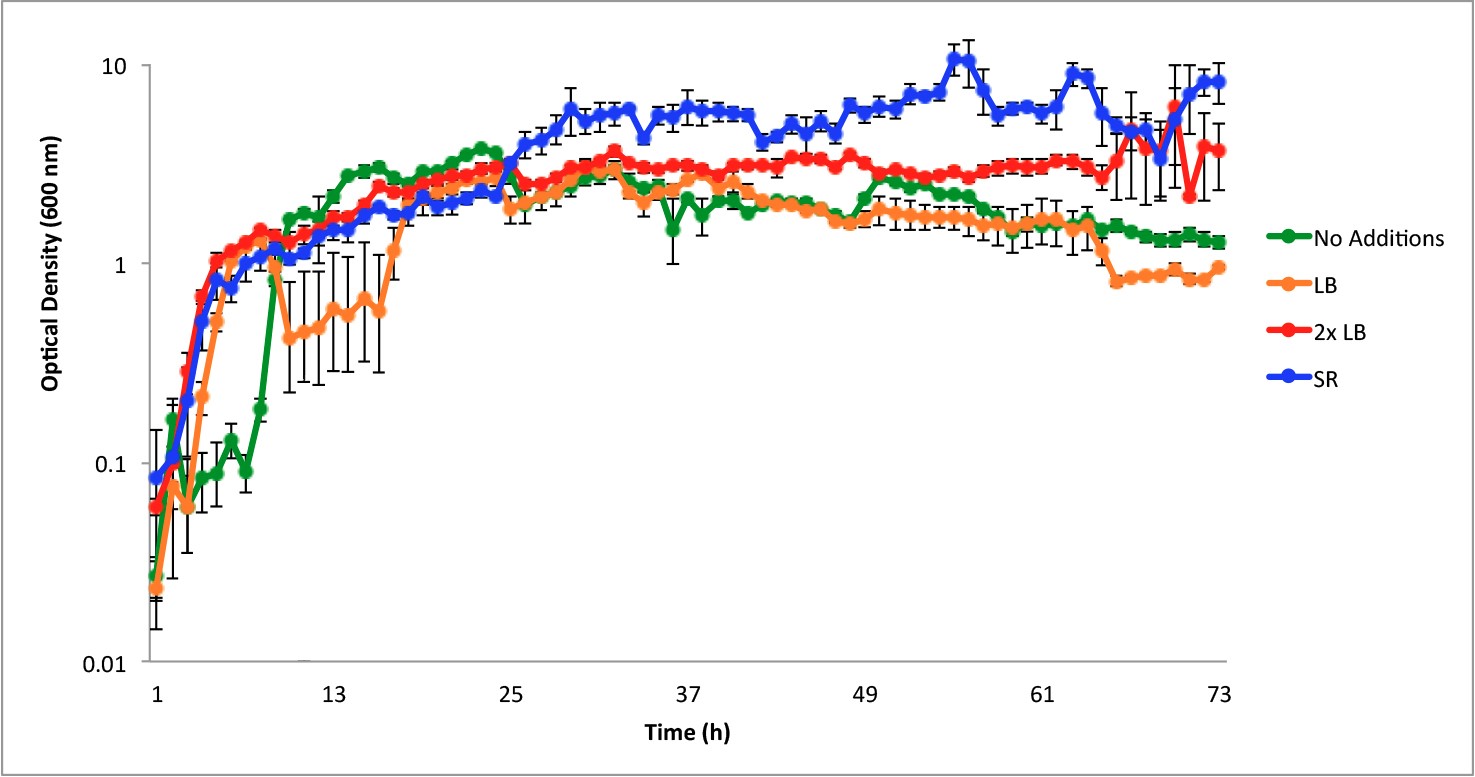
Figure 3 shows the growth curves of B. subtilis WB800 at 35°C in LB with the periodic addition of one mL aliquots of 3 different types of media (LB, 2X concentrated LB and SR media). The greatest amount of growth was observed in the cultures receiving SR media, where the addition of SR media every 12 hours extended the growing phase to 72 hours. This is in contrast to the growth curves when either LB or no media was added, both of which begin to show a downward trend in growth after about 24 hours. When B. subtilis WB800 was grown with periodic addition of 2X LB, the optical density increased, but did not reach values as high as cultures grown with addition of SR medium.
mBBI Decreases the Amount of Double-Stranded Breaks in Primary Fibroblast Cells
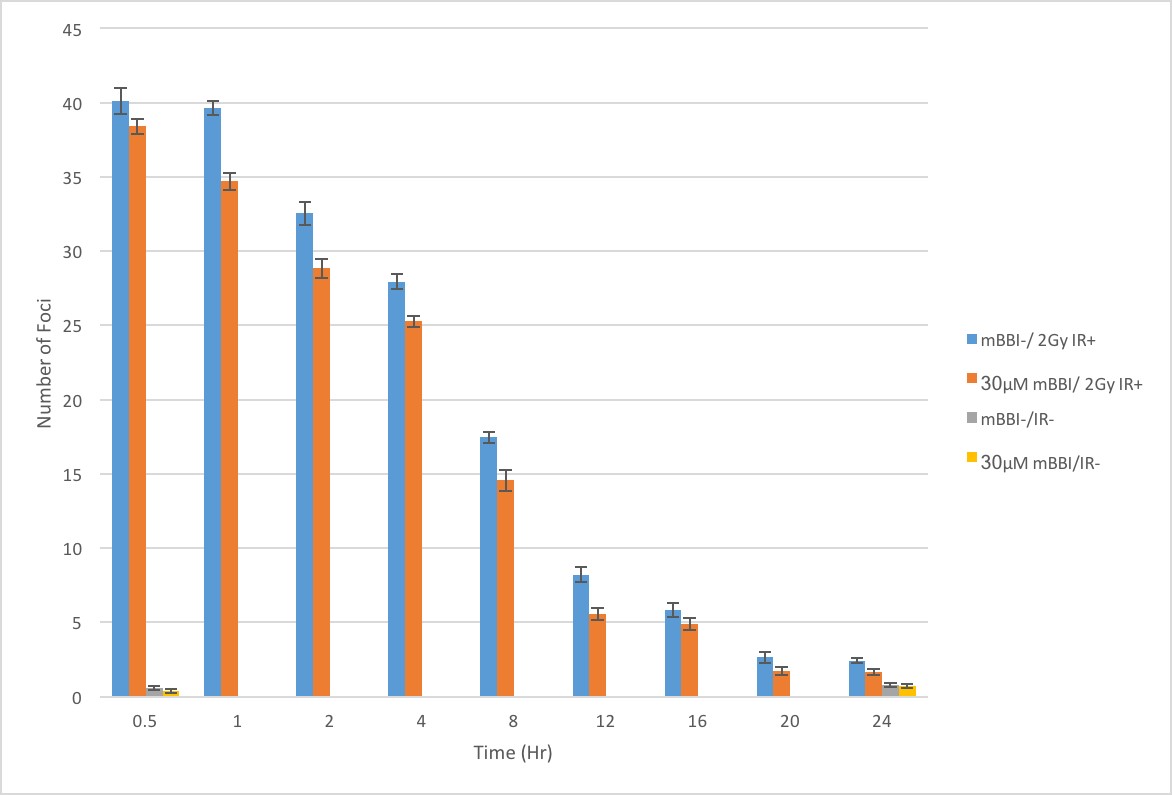
Whether cells were pretreated or not with mBBI prior to IR exposure, they expressed similar numbers of γH2AX and 53BP1 foci immediately following exposure to IR (Figure 4). Both cell treatments showed reductions in the number of γH2AX and 53BP1 foci over the following 24 hours, with the curve resembling an inverse decay. However, with mBBI pretreatment, cells showed up to a 32% decrease in the number of foci consistently across all time points following IR exposure compared to untreated cells (Figure 4). Cells pretreated with mBBI without IR exposure showed no increases in foci at either 0 or 24 hours when compared to the double negative control. No significant difference was observed in the amount of double-stranded breaks in the cells pretreated with oxidized and reduced mBBI (data not shown).
Containment of B. subtilis within the Transdermal Patch
Green fluorescent protein-expressing bacteria in LB were placed in a syringe whose outlet was fitted with either the EVA membrane or a 0.2-micron filter sterilization membrane, and the whole was immersed in a saline solution. Aliquots of the saline solution were taken at 24-hour intervals and plated to determine whether bacteria had escaped across the membrane. Figure 5 shows plates of aliquots taken at Day 7 seen under UV light. The plated aliquot of the saline solution from the EVA filter syringe set-up showed growth of the GFP-expressing bacteria (top left). No growth was observed in the plates of aliquots taken from the 0.2-micron filter set-up (top right), and none from the control (bottom). The same results were obtained from days 1 to 6.

Transdermal Patch Prototyping and Peptide Diffusion Model
Figure 6 illustrates a graph depicting the expected concentration of peptide in the blood over time based upon a modified version of Fick’s Law of Diffusion. It shows that based on the current design considerations, it would take a considerable time for the concentration of peptide to increase, and it does not reach a steady concentration within the 60-hour time constraint of the model. The steady state time could be calculated by setting equations 4, 6 and 8 to equal 0.

Human Practices
Policy Concerning the Regulation of Biotherapeutics in Canada
The evolving fields of synthetic biology and genetic engineering allow for the creation of innovative cell-based solutions only restricted by the imagination. The development of mBBI as a remedy to long-term IR exposure is one example. However, the ease of manipulation of organisms for new applications may pose risks to the sustainability, biosafety, biosecurity, ethics, and design efficacy of therapeutic synthetic biology products. Due to the rapid development of innovative drugs produced by and harvested from living organisms (hereby referred to as biotherapeutics) in the consumer market in recent years, Canadian policy and regulations will soon fail to properly cover the entire collection of biotherapeutic products, which do not resemble products on which existing regulations have been based.[24] Currently, the safe manufacture and distribution of biotherapeutics like mBBI in Canada is mandated by the Canadian Food and Drugs Act of 1985.[25] Enforcement of this act and implementation of specific guidance for each drug type is overseen by Health Canada. These regulations were created assuming that biotherapeutics and synthetic biology products could be compared and handled in similar ways to products of fields such as synthetic chemistry and traditional genetic engineering, which involves introduction of only one or a few genes into an organism’s genome.[26] As a result, risks assessments and containment strategies fail to account for the unique properties of biotherapeutics. Problems will arise when biotherapeutic products can no longer be accurately assessed and regulated with existing guidelines and policies.
It is recommended that specific guidelines and regulations for the advancement of the field of biotherapeutics be created, especially for novel and innovative biotherapeutic products, such as the development of an mBBI transdermal patch where no specific legislation applies. The introduction of such specific laws and regulations will allow for the safe research and use of biotherapeutics. For these regulations to be effective, they need the coordinated efforts of companies, academic institutions, and other partners. They must build off of existing framework, as they require support from international institutions that are currently involved first-hand in developing biotherapeutics. Strategies attempting to formulate regulations of biotherapeutics should include an adaptive drug licensing process that takes into consideration the current existing standard indicators used by researchers.
Discussion
There are two fields in which radiation damage is a concern: medicine (radiotherapy) and space travel. To determine the best application for a radioprotective device, experts in the field of cancer research and treatment were consulted, including Dr. Corinne Doll, Dr. Eduardo Villarreal-Barajas, and Dr. Nicholas Ploquin. Based on these interviews it was determined that the patch should focus on protecting astronauts rather than cancer patients, since improved dosing and stereotactic delivery have reduced the risk of secondary malignancies incurred from radiation therapy.
Three types of devices for delivery of a peptide were considered with the application of space travel in mind: Injections, transdermal patches, and implantable devices. Following consultation with CSA astronauts LCol Jeremy Hansen and Dr. Robert Thirsk, CSA operational space medicine project officer Dr. Leena Tomi, and NASA astronaut Dr. Yvonne Cagle, the transdermal patch was found to be the most suitable given its properties: patches are lightweight, non-invasive, and easy to apply and store. These are all features that make a patch ideal for space missions.
The safety of the patch was considered in both the choice of patch materials and the choice of bacterial chassis following conversations with Dr. Craig Jenne, Dr. Colin Dalton, Dr. Hans Vogel, and Dr. Elke Lohmeier-Vogel.
Genetic Circuit Information
The genetic circuits were designed around the central theme of transdermal diffusion and peptide production. The addition of a transdermal-1 (TD1) tag to the N-terminus of mBBI was used to promote the movement of the peptide across the various skin layers and into the circulatory system.[16] To ensure secretion of the peptide, a secretory (sec) tag was fused upstream of the TD1 tag. This secretary tag is a short amino acid sequence native to B. subtilis and functions as a signal for the bacterium to export the peptide to the plasma membrane for transport out of the cell, where the sec tag is cleaved from the peptide sequence.[27] The addition of the sec tag allowed for the exploitation of an already-existing secretory system in B. subtilis.
The production of mBBI had two requirements: (1) the continuous production of mBBI; and (2) the permanent transformation of B. subtilis cells. To ensure continuous production of mBBI, a constitutively active promoter from B. subtilis was integrated into the genetic circuits. The promoter, PVeg, was identified as being suitable. This promoter was identified from the iGEM Registry of Standard Biological Parts as a regulatory sequence whose strong expression had been documented by several different iGEM teams and in literature.[28,29] The second element included in the genetic circuits for protein production were sequences homologous to B. subtilis genes. The inclusion of sequences for AmyE and thrC loci both upstream and downstream of the mBBI sequence would allow for the homologous recombination of the genetic constructs with the B. subtilis genome and integration of the mBBI gene into the genome. This integration would be necessary to ensure that the transformation of B. subtilis would not be transient.
The final element of the genetic circuits was the inclusion of comK, the master control gene for competency.[12] The comK gene was placed under the control of a xylose-inducible promoter, PXylA in order to improve transformation efficiency.[30]
An important feature of the genetic circuit design is that the mBBI insert can easily be released by restriction enzyme digestion and replaced with a sequence coding for a new biotherapeutic. With comK, the efficiency of transformation of B.subtilis with a new genetic construct would be similarly enhanced. The expression and delivery of the new biotherapeutics would be facilitated by the same elements that favor mBBI expression and delivery: the transdermal and the secretion tags. Thus, the versatility of the design of the genetic circuit would allow for the substitution of different biotherapeutics in place of mBBI to enhance the functionality and usability of the patch system being developed.
Growth Curve Review
The bacterial growth curve indicated the temperature at which B. subtilis will display the fastest growth. The highest population density was achieved at 35°C (Figure 2). These results were expected given that B. subtilis is a part of the natural skin microflora.[31] This growth curve displayed a clear exponential phase from 1-8 hours and stationary phase of growth at 13-17 hours when bacteria were incubated at 35°C. While both cultures grown at 35°C and 22°C reached similar levels of growth after 24 hours, the 35°C treatment would be more beneficial within the context of a transdermal drug patch, since the cultures at 35°C were able to enter the log phase within a shorter time. The more rapid entry into log phase would favor adequate peptide production, as that is time period where the greatest amount of peptide would be produced.[32] The rapid entry into log phase could be coupled with having a higher initial cell density to maximize the number of cells that would produce and secrete mBBI. However, after 17 hours, the B. subtilis population at 35°C started to decrease due to a lack of nutrients.
To determine whether sustained growth could be achieved within the patch system for its extended use, 1-mL additions of different media were added to the bacterial cultures grown at 35°C every 12 hours for the first 36 hours. When additional media was supplemented every 12 hours, the stationary phase was maintained for over 72 hours (Figure 3). The highest growth was observed in SR, while 2XLB media closely followed. The SR medium was able to foster greater growth due to the doubled amount of yeast extract and the addition of glucose relative to 2XLB.[19] From 0-23 hours, there was an overlap between the SR and 2XLB treatments; however, this does not affect the outcome of the experiment since the purpose was to determine which of the media have the greatest benefit when supplemented over the long term. This growth curve indicated that B. subtilis populations could be sustained over 72 hours if the media was periodically supplemented with fresh nutrients, and this informed the design of the patch to include extra media packets.
Radioprotective Effects of mBBI
Following irradiation, protein complexes form adjacent to the site of double strand breaks. Proteins, such as 53BP1, can be stained with fluorescent antibodies to acquire a visual representation (foci) of DNA double strand breaks. The decrease in the number of 53BP1 foci over time is indicative of DNA repair. The preliminary data indicates that the pretreatment of 1BR3 cells with mBBI results in a reduction of 53BP1 foci faster than basal repair rates, following 2 Gy of gamma radiation exposure (Figure 4). The initial numbers of double-stranded breaks were observed to be similar (4% difference), however, the subsequent numbers dropped by up to 32% at later time points. This indicates that mBBI treated cells have faster rates of DNA repair, given both treatment groups had approximately equal amounts of double-stranded breaks at time t=0. This trend was consistent with past observations in other cell types showing faster foci reduction and increased viability of cells treated with mBBI after IR exposure.[5,33] It has been proposed that BBI enhances repair kinetics by inducing EGFR internalization and priming the cell for DNA repair by activating DNA PK. Our data supports this hypothesis by indicating that pretreatment with mBBI results in more rapid loss of double-stranded break foci. The activation of DNA PK resulting from mBBI pretreatment causes the earlier initiation of repair and faster repair of double-stranded breaks. Thus it can be argued that mBBI does not protect the cell from IR; rather, it primes the cell’s natural DNA repair mechanism.
Patch Design
The equations for the diffusion model were solved using MATLAB. Figure 6 illustrates a MATLAB plot of the concentration of peptide over time in the blood. This graph shows that the peptide would not reach a constant concentration in the blood while the patch is in use over 60 hours. The time was extended to determine when the peptide concentration in the blood would reach a steady state, and this was found to be at about four weeks, which in practice would be of little use. Figure 6 also shows that within 60 hours, the peptide concentration will not be expected to reach 10 μM, the minimum needed for mBBI to confer radioprotection.[6] Both the time to steady state and the indication that the 10 μM serum concentration will not be achieved suggest that much still needs to be done. The materials used in the patch should be reconsidered, e.g., a size-controlling membrane with a higher diffusion coefficient needs to be investigated. Other design considerations include finding a way to increase peptide production above the assumed output of 1 mg/ml, and extending the lifetime of engineered B. subtilis WB800 so that the peptide is produced for longer periods of time.
Materials were chosen to fit specifications suggested by astronauts from the CSA and the NASA. In order to be compatible with missions in space, a device must be lightweight and non-invasive, easy to use, produce little waste, and easy to store for longer intervals. As such, the transdermal patch designed in this study befits the criteria necessary for space travel.
Several engineering controls were incorporated in the patch to combat contamination issues in different environments such as on Earth, in transit, and in space. The results of the patch diffusion assay showed that EVA membrane by itself would not be able to contain the bacterial cells, since GFP-transformed bacteria were observed on plated samples of the saline solution from the EVA membrane. In contrast, the diffusion assay conducted with 0.2-micron filter sterilization membrane (Pall Corporation) demonstrated that it could contain the bacterial cells, as indicated by the absence of growth on the plated samples. Thus, a 0.2-micron filter was incorporated into the original design of the patch as a secondary membrane. This would ensure further filtering by the patch to prevent contamination. Additional engineering controls included the use of a backing layer to provide structural support and resistance to damage from physical impact on the patch.
Future Directions
Future experiments would involve replacing the constitutive promoter; PVeg with a promoter such as PohrB, which is inducible by relatively simple means, i.e., by heat shock or glucose deprivation.[34] This replacement would be expected to improve the yield of peptide since this promoter has been shown to initiate gene expression at greater than basal levels.
Ensuring the integration of the synthetic comK circuit into the B. subtilis chromosome at multiple loci would be worth pursuing. Having comK, the master regulatory gene of competency, under the control of an inducible promoter would allow for the increased efficiency of transformation of the B.subtilis, providing the opportunity for the future exchange of biotherapeutic inserts.[12] For instance, the mBBI coding sequence could be replaced with another biotherapeutic such as one that would increase radiosensitivity. The transdermal delivery of this new biotherapeutic would take advantage of the features that have been built into the original genetic construct’s design. Being able to quickly manipulate B. subtilis to produce other biotherapeutics could transform the patch system into an efficient mobile molecular pharmacy.
For safety purposes, an auxotrophic B. subtilis strain, such as lysA knockout, would be engineered to prevent the proliferation of bacteria should it diffuse through the semipermeable membrane. The gene product of the lysA is responsible for the decarboxylation of meso-diaminopimelate to L- lysine.[35] A knockout B. subtilis WB800 strain of lysA would not be able to successfully propagate without the addition of L-lysine, confining the bacteria to the patch. Additionally, integrating sequences encoding mBBI in various other parts of the B. subtilis genome which are essential for amino acid synthesis like lysA could provide more opportunities for auxotrophy, increasing the safety of using B. subtilis in the device, while increasing peptide yield. The media in the transdermal patch would then contain the required amino acids with respective to the auxotrophy, which will ensure that the bacteria can only successfully proliferate inside the patch.
To further characterize the action of mBBI, testing is required in human cell lines other than primary human fibroblasts. Additionally, clonogenics testing would be useful to characterize the effect that mBBI pretreatment and the reduction of double-stranded breaks has on total cell survival after irradiation.
In order to make the diffusion model more accurate, the diffusion of the peptide through individual layers of the skin will be incorporated into the model. Moreover, the model will also consider the transdermal tag TD1, which would increase the diffusion rate of the peptide. Bench data on measures of actual peptide output from the bacteria would be extremely helpful in the iterative refinement of the model.
These modifications to the model will help to determine (1) whether the peptide reaches a steady concentration in the blood, (2) what that steady concentration is, (3) the time it takes to reach the steady concentration, (4) the concentration at different locations in the skin and body at any given time point, and (5) effects on mBBI concentration in the blood when the patch is removed. The last point is important to consider, as it will help answer questions such as when the user should apply a new patch. A reverse of the model could also be developed to find the initial concentration of mBBI in the patch to achieve the radioprotective dose of 10 μM.[6] The results of this would inform experiments on the wet bench, to work toward achieving the target initial concentration so identified.
In conjunction with the results of the patch diffusion assay and initial math model, further material testing is required to ensure survivability of cells, and monitor diffusivity of mBBI. Mechanical testing, such as finite element analysis, on the prototype design is also required to ensure durability of the patch in storage and during wear. This testing will provide a method to identify areas of weakness in the patch when subjected to different loading scenarios and in different environmental conditions. Mechanical analysis will also aid in identifying a design that maximizes the diffusion of mBBI.
Conclusions
Ionizing radiation is a barrier that must be overcome to allow for long term space missions, as it causes double-stranded breaks in DNA, which can result in harmful mutations and increase lifetime risk of cancer. One potential solution is to enhance existing DNA repair mechanisms. The modified nonamer (mBBI) of the BBI peptide has been found to enhance natural double-stranded break repair pathways in cells, and may thus be able to mitigate damage from ionizing radiation exposure. Through an H2AX assay, BBI was found to decrease the number of double-stranded breaks in cell populations exposed to ionizing radiation. A patch was designed that would contain B. subtilis WB800 engineered to produce and secrete a modified form of the BBI peptide, mBBI, which was tagged with both secretion tags and a transdermal tag. In the design, bacteria are contained within a chamber of the patch and supplied with growth media that can be replenished by popping additional packets of media. A size and rate controlling membrane ensures that bacteria are contained within the patch but mBBI is able to travel through the skin into the circulatory system. In the diffusion assay, it was determined the EVA membrane (3M) did not prevent the diffusion of bacterial cells. Thus, a 0.2-micron filter membrane (Pall) was incorporated into the design as a secondary membrane to ensure bacterial containment. The patch was designed to meet specifications for space travel following consultation with astronauts and other professionals. A diffusion model was also developed to provide qualitative answers to key questions such as the expected serum concentration of BBI over time. The predictions of the present model suggest that the design and materials used in the patch need to be reconsidered, and must take into account data that would come from laboratory experiments, in order to refine the model.
Acknowledgments
We would like to thank all the members of the Goodarzi, Lees-Miller, and Jenne labs who supported us in this project, as well as members of the Schulich School of Engineering Machine Shop. In particular, we would like to thank Dr. Aaron Goodarzi, Dr. Susan Lees-Miller, and Dr. Craig Jenne for their mentorship and guidance. We would also like to acknowledge the continual support of Dr. Sui-Lam Wong for the valuable assistance on our work with B. subtilis. We appreciate the guidance of Dr. Michael Kallos, Dr. Colin Dalton, Dr. Justin MacCallum, Dr. Amir Sanati Nezhad, Dr. Uttandaraman (U.T.) Sundararaj, Robert Mayall, Peter Byrne, and Tylor Walsh on the design and modelling of the transdermal patch. We would like to thank Dr. Elke Lohmeier-Vogel, Dr. Hans Vogel, and Dr. Wendy Hutchins for their expertise and knowledge in the field of biochemistry and troubleshooting of mBBI-related experiments. The guidance of Dr. Agnes Klein, Dr. Fabiola Aparicio-Ting, Dr. Walter Glannon, Dr. Gregory Hagen, Dr. Eduardo Villarreal-Barajas, Dr. Nicolas Ploquin was greatly appreciated in the design of our product for end-users. Consultation with CSA astronauts Dr. Robert Thirsk and LCol Jeremy Hansen, CSA operational space medicine project officer Dr. Leena Tomi, and NASA astronaut Dr. Yvonne Cagle was appreciated to arrive at a final patch compatible for use in space. Our gratitude is further extended to all supporters of our project endeavors.
Response to Reviewers
A transcript of the reviewer comments and author responses from the Live Peer Review Jamboree can be found here: UCalgary Response to Reviewers
References
- Durante M, Cucinotta, FA. Physical Basis of radiation protection in space travel. Rev Mod Phys 2011 Aug;83(4):1245–1281.
- Lees-Miller SP, Meek K. Repair of DNA double strand breaks by non-homologous end joining. Biochimie 2003 Nov;85(11):1161–1173.
- Frazier S. Edited by Garner, R. Real Martians: How to Protect Astronauts from Space Radiation on Mars [Internet]. NASA: Journey to Mars Feature 2015. [cited 17 Jan 2017]. Available from: https://www.nasa.gov/feature/goddard/real-martians-how-to-protect-astronauts-from-space-radiation-on-mars.
- Dunbar B. Advanced Space Transportation Program: Paving the Highway to Space [Internet]. NASA. Marshall Space Flight Centre; 2004 [cited 17 Jan 2017]. Available from: https://www.nasa.gov/centers/marshall/news/background/facts/astp.html.
- Dittmann K, Mayer C, Kehlback R, Rodemann HP. The radioprotector Bowman-Birk proteinase inhibitor stimulate DNA repair via epidermal growth factor receptor phosphorylation and nuclear transport. Radiother Oncol 2008;86(3):375–382.
- Dittmann KH, Gueven N, Mayer C, Ohneseit P, Zell R, Begg AC, et al. The Presence of Wild-Type TP53 Is Necessary for the Radioprotective Effect of the Bowman-Birk Proteinase Inhibitor in Normal Fibroblasts. Radiati Res 1998;150(6):648.
- Dittmann KH, Gueven N, Mayer C, Rodemann HP. Characterization of the amino acids essential for the photo- and radioprotective effects of a Bowman-Birk protease inhibitor-derived nonapeptide. Protein Eng 2001;14(3):157–160.
- Prausnitz MR, Langer R. Transdermal drug delivery. Nature biotechnology. 2008;26(11):1261–1268.
- Wu SC, Yeung JC, Duan Y, Ye R, Szarka SJ, Habibi HR, Wong SL. Functional production and characterization of a fibrin-specific single-chain antibody fragment from Bacillus subtilis: effects of molecular chaperones and a wall-bound protease on antibody fragment production. Appl Environ Microbiol 2002 Jul;68(7):3261–3269.
- McKenney PT, Driks A, Eichenberger P. The Bacillus subtilis endospore: assembly and functions of the multilayered coat. Nat Rev Micro. 2013 Jan;11(1):33–44.
- Yamane K, Bunai K, Kakeshita H. Protein Traffic for Secretion and Related Machinery of Bacillus subtilis. Bioscience, Biotechnology, and Biochemistry. 2004 Jan 1;68(10):2007–23.
- van Sinderen D, Luttinger A, Kong L, Dubnau D, Venema G, and Hamoen L. comK encodes the competence transcription factor, the key regulatory protein for competence development in Bacillus subtilis. Mol Microbiol. 1995 Feb;15(3):455–462.
- Bekker-Jensen S, Lukas C, Kitagawa R, Melander F, Kastan MB, Bartek J, et al. Spatial organization of the mammalian genome surveillance machinery in response to DNA strand breaks. J Cell Biol 2006;173(2):195–206.
- Mah L-J, El-Osta A, Karagiannia TC. γH2AX: a sensitive molecular marker of DNA damage and repair. Laukemia 2010;24(4):679–686.
- Löbrich M, Shibata A, Beucher A, Fisher A, Ensminder M, Goodarzi AA, et al. γH2AX foci analysis for monitoring DNA double-strand break repair. Cell Cycle 2010;9(4)662–669.
- iGEM USTC_CHINA 2013. USTC_CHINA 2013 Parts. In: USTC_CHINA 2013 [Internet]. 2013 [cited 17 Jan 2017]. Available from: http://2013.igem.org/Team:USTC_CHINA/Parts.
- iGEM UofC_Calgary 2016. UofC_Calgary 2016 Composite Parts. In: UofC_Calgary 2016 [Internet]. 2016 [cited 17 Jan 2017]. Available from: http://2016.igem.org/Team:UofC_Calgary/Composite_Part.
- iGEM UofC_Calgary 2016. UofC_Calgary 2016 Experiments. In: UofC_Calgary 2016 [Internet]. 2016 [cited 17 Jan 2017]. Available from: http://2016.igem.org/Team:UofC_Calgary/Experiments.
- Halling SM, Sanchez-Anzaldo FJ, Fukuda R, Doi RG, Meares CF. Zinc Is Associated with the β Subunit of DNA-Dependent RNA Polymerase of Bacillus subtilis. Biochem 1977 June;16(1):2880–2884.
- Arlett CF, Harcourt SA. Survey of radiosensitivity in a variety of human cell strains. Cancer Res 1980;40:926–932.
- Hosoya O, Chono S, Saso Y, Juni K, Morimoto K, Seki T. Determination of diffusion coefficients of peptides and prediction of permeability through a porous membrane. J Pharm Pharmacol 2004;56(12):1501–1507.
- Gibney MA, Arce CH, Byron KJ, Hirsch LJ. Skin and subcutaneous adipose layer thickness in adults with diabetes at sites used for insulin injections: implications for needle length recommendations. Curr Med Res Opin 2010;26(6):1519–1530.
- Westers L, Westers H, Quax W. Bacillus subtilis as cell factory for pharmaceutical proteins: a biotechnological approach to optimize the host organism. Biochimica Et Biophysica Acta (BBA) – Mol Cell Res 2004;1694(1-3):299–310.
- Fischbach, M.A., Bluestone, J.A., Lim, W.A. Cell Based Therapeutics: the Next Pillar of Medicine. Sci Transl Med. 2013;5(179):7–19.
- Government of Canada. Food and Drugs Act. R.S.C. 1985. c. F-27. [cited 20 Jan 2017]. Available from: http://laws-lois.justice.gc.ca/eng/acts/F-27/FullText.html.
- Epstein, M.M., Vermiere, T. Scientific Opinion on Risk Assessment of Synthetic Biology. Trends in Biotechnology 2016;34(8):601–603.
- Fu LL, Xu ZR, Li WF, Shuai JB, Lu P, Hu CX. Protein secretion pathways in Bacillus subtilis: Implication for optimization of heterologous protein secretion. Biotechnology Advances. 2007 Jan;25(1):1–12.
- Part:BBa K823003 – parts.igem.org [Internet]. [cited 2017 Jan 21]. Available from: http://parts.igem.org/Part:BBa_K823003.
- Lam KHE, Chow KC, Wong WKR. Construction of an efficient Bacillus subtilis system for extracellular production of heterologous proteins. Journal of Biotechnology. 1998 Aug 27;63(3):167–77.
- Zhang XZ, Zhang Y-HP. Simple, fast and high-efficiency transformation system for directed evolution of cellulase in Bacillus subtilis. Microbiol biotech. 2011 Jan;4(1):98–105.
- Ara K, Hama M, Akiba S, Koike K, Okisaka K, Hagura T, et al. Foot odor due to microbial metabolism and its control. Can J Microbiol. 2006 Apr 1;52(4):357–64.
- Rosano GL, Ceccarelli EA. Recombinant protein expression in Escherichia coli: advances and challenges. Front Microbiol 2014 Apr;5:172.
- Dittmann K, Virsik-Köpp P, Mayer C, Rave-Fränk M, Rodemann HP. Bowman-Birk protease inhibitor activates DNA-dependent protein kinase and reduces formation of radiation-induced dicentric chromosomes. Int J Radiat Biol 2003 Oct;70(10):801–808.
- Panahi R, Vasheghani-Farahani E, Shojaosadati, SA, Bambai, B. Auto-inducible expression system based on the SigB-dependent ohrB promoter in Bacillus subtilis. Mol Biol (Mosk). 2014; 48(6):970–976.
- UniProt. UniprotKB-P23630 (DCDA_BACSU). 2017 [cited 20 Jan 2017]. Available from: http://www.uniprot.org/uniprot/P23630.